UNDERSTANDING WOMEN’S NEEDS
Approximately 65% of American women of reproductive age use some form of contraception, but many may have not found a contraceptive solution that fully meets their needs.1
The American College of Obstetricians and Gynecologists recommends Long-Acting Reversible Contraception (LARC), such as intrauterine devices (IUDs), as a safe and effective contraceptive option for women, including those who have not given birth and adolescents who are sexually active. The use of IUDs in the U.S. has been increasing, yet there has been little product innovation in this category.2-4
ELEVATING THE IUD EXPERIENCE
Sebela Women’s Health® is developing a next-generation IUD platform with products designed to fit the uniqueness of every woman’s body. Developmental features of this IUD platform are a flexible nitinol frame, a preloaded inserter, a smaller insertion tube, and precut retrieval strings. It’s the only platform that will include both a hormonal and hormone-free IUD option—because we understand that women have different needs and preferences. With our innovative portfolio, we seek to offer an improved IUD experience for women and HCPs.
ONE PLATFORM, TWO IUD OPTIONS

MIUDELLA®
At a time when demand for hormone-free options is growing rapidly, Sebela has developed the first hormone-free intrauterine system (IUS) for the U.S. market in four decades. MIUDELLA® is a lower-dose copper intrauterine device that has recently been approved by the FDA for prevention of pregnancy in females of reproductive potential for up to 3 years. Clinical trials remain ongoing to evaluate efficacy and safety for up to 8 years of use. 5-7
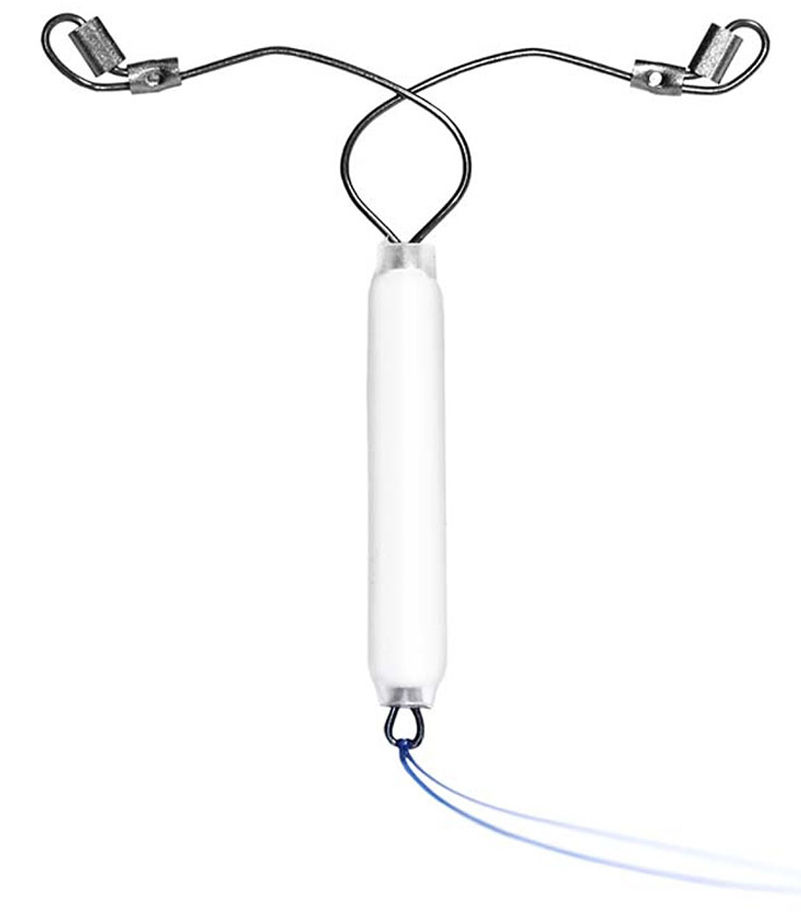
52 mg Levonorgestrel IUD
We understand that many women who are looking for highly effective pregnancy prevention also appreciate the non-contraceptive benefits afforded by hormone-based contraceptives. That’s why we’re developing an IUD with the advanced design features and benefits of the Sebela platform and a Levonorgestrel reservoir.
52 mg Levonorgestrel IUD is Phase 3 ready. It is limited by United States Federal Law to investigational use.8
References
1. Daniels K, Abma JC. Current contraceptive status among women aged 15–49: United States, 2015–2017. NCHS Data Brief, no 327. Hyattsville, MD: National Center for Health Statistics. 2018.
2. The American College of Obstetricians and Gynecologists. Practice Bulletin. Long-acting reversible contraception: implants and intrauterine devices. Accessed February 13, 2025. https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2017/11/long-acting-reversible-contraception-implants-and-intrauterine-devices
3. Guttmacher Institute. Contraceptive Use in the United States by Method. Accessed October 7, 2022. https://www.guttmacher.org/fact-sheet/contraceptive-method-use-united-states
4. Nelson AL, Massoudi N. New developments in intrauterine device use: focus on the US. Open Access J Contracept. 2016;7:127-141. doi:10.2147/OAJC.S85755
5. ClinicalTrials.gov. Evaluation of Efficacy, Safety, and Tolerability of VeraCept IUD. Accessed February 14, 2024. https://clinicaltrials.gov/study/NCT03633799
6. MIUDELLA® [package insert]. Roswell, GA: Sebela Women’s Health Inc.
7. U.S. Food and Drug Administration. Technical Considerations for Non-Clinical Assessment of Medical Devices Containing Nitinol Guidance for Industry and Food and Drug Administration Staff. Accessed February 14, 2025. https://www.fda.gov/media/123272/download.
8. ClinicalTrials.gov. Evaluation of the Efficacy, Safety, and Tolerability of LevoCept. Updated March 5, 2024. Accessed February 13, 2025. https://clinicaltrials.gov/ct2/show/NCT04457076